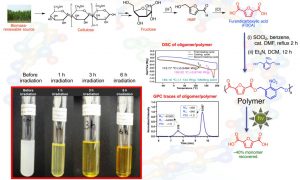
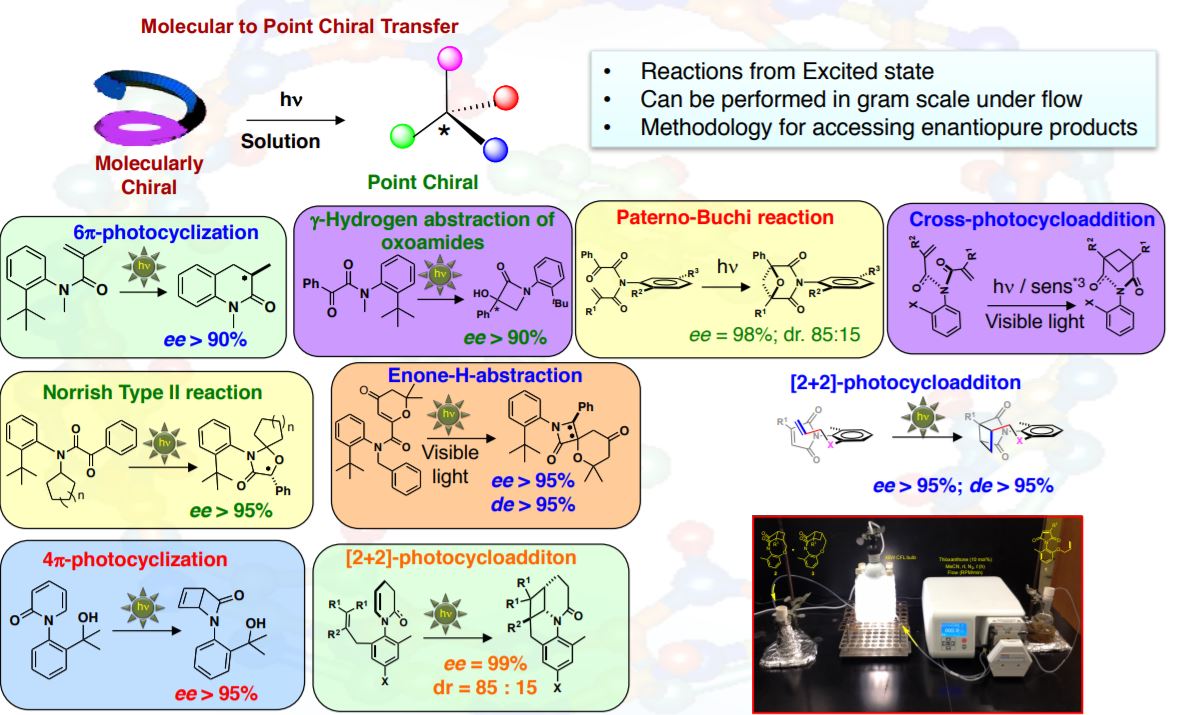
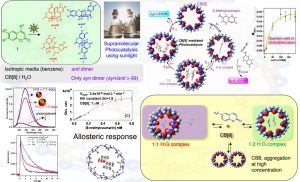
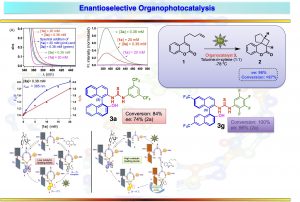
Photophysics
Using photo physical tools our group we understand excited state processes to solve mechanistic puzzles, unravel new reactivity, develop new chemical paradigms from excited states and develop new systems that are responsive to light. Our group pursues both steady state and time resolved techniques to address research challenges that originate from excited state reactivity.
- Iyer, A; Clay, A. C.; Jockusch, S.; Sivaguru, J.*.Evaluating brominated thioxanthones as organo-photocatalysts. J. Phy. Org. Chem., 2017, 30:e3738. (Waldemar Adam Special Issue)
- Wang, W; Clay, A; Krishnan, R; Lajkiewicz, N; Sivaguru, J;* Porco, J* Total Syntheses of the Aglain Natural Products Foveoglin A and Perviridisin B via selective ESIPT Photocycloaddition. Angew. Chem. Int. Ed., 2017,56, 14479 –14482.(accepted; DOI: 10.1002/anie.201707539 and 10.1002/ange.201707539). (Highlighted in Synfacts2018, 14, 0005).
- Brown, S. L.; Krishnan, R.; Elbaradei, A.; Sivaguru, J.; Sibi, M. P.; Hobbie, E. K.“Origin of Stretched-Exponential Photoluminescence Relaxation in Size-Separated Silicon Nanocrystals,AIP Advances, 2017, 7, 55314.
- Srinivasan, G.; Hoey, J.; Anderson, K.; Frohlich, M. T.; Krishnan, R.; Sivaguru, J.; Sibi, M. P. Boudjok. P. “Synthesis of Silicon Quantum Dots using Cyclohexasilane (Si6H12)” J. Mater. Chem. C, 2016, 4, 8206-8213.
- Clay, A.; Vallavoju, N., Krishnan, R.; Ugrinov. A.; S., J. Sivaguru*Metal Free Visible Light Mediated Photocatalysis: Control-ling Intramolecular [2+2] Photocycloaddition of Enones through axial chirality. J. Org. Chem. 2016, 81, 7191−7200. (Special issue on Photocatalysis).
- Vallavoju, N.; Sreenitya, A.; Ayitou, A.; Jockusch, S.; Sunoj, R. B.; and Sivaguru, J.* Photoreactions with a twist: Atropisomerism-driven divergent reactivity of enones with UV and visible light. Chem. E. J.2016, 22, 11339-11348.