Organic Photochemistry
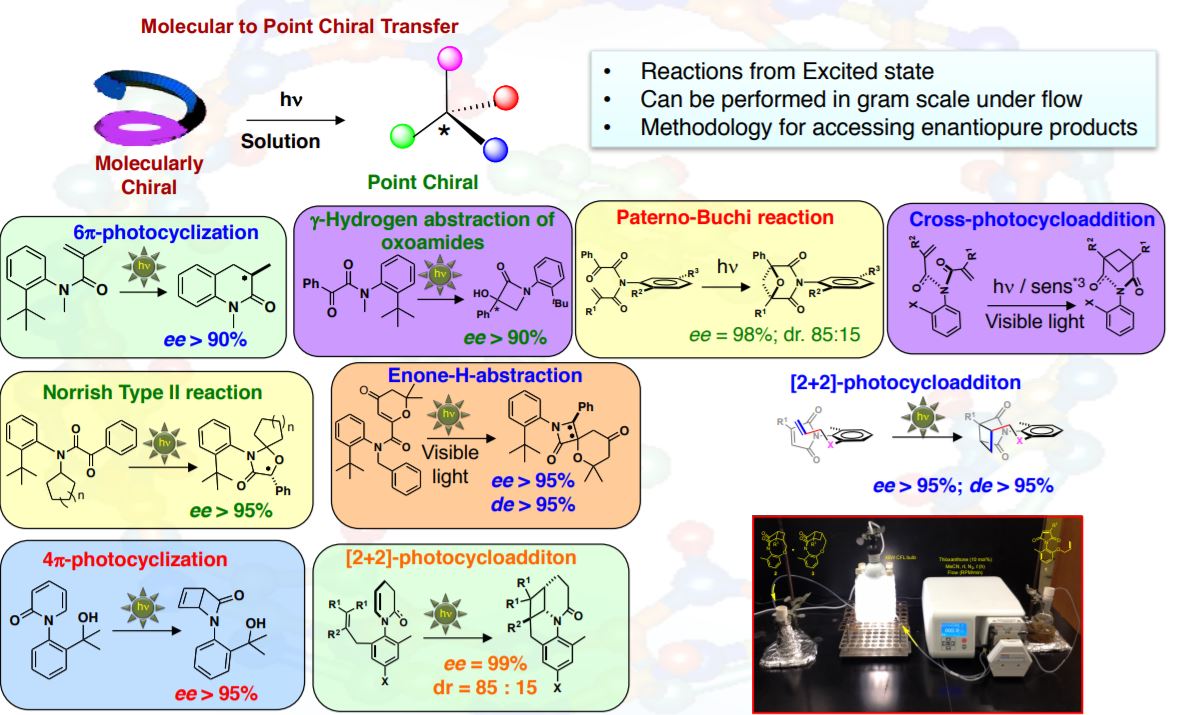
We have established the importance of restricted bond rotations in controlling excited state reactivity. Utilizing atropisomeric scaffolds we have unraveled new design features, with outcomes that are unique and unprecedented for excited state reactivity. For example, we have established that reactive spin states (singlet or triplet excited state) profoundly influence the stereochemical outcome of phototransformations. We have also detailed the use of low energy visible light rather than high energy UV light without compromising the stereoenrichment in the photoproducts. In general, the photochemistry and photophysics of atropisomeric substrates differ significantly from their achiral counterparts irrespective of having the same chromophore initiating the excited state reactivity. The generality and versatility of our strategy was detailed as an accounts of chemical research article signifying the scope of our strategy in accessing chirally enriched products during phototransformations. Some recent references related to our group’s contribution to organic and asymmetric photochemistry are below.
- Kumarasamy, E; Ayitou, A. J. -L.; Vallavoju, N.; Raghunathan, R.; Iyer, A.; Clay, A.; Kandappa, S.; Sivaguru, J.* Tale of Twisted Molecules: Taming Asymmetric phototransformations through non-biaryl atropisomers. Acc. Chem. Res. 2016. 49, 2713−2724.
- Kumarasamy, E; Raghunathan, R.; Jockusch, S.; Sivaguru, J.* TransposedPaternò-BüchiReaction J. Am. Chem. Soc., 2017, 139, 655–662.
- Iyer, A; Jockusch, S.; Sivaguru, J.* Making the transition– Enablingtraditionalphotoreactionstoworkundervisiblelight. Chem. Commun. 2017, 53,1692-1695.
- Kumarasamy, E.; Kandappa, S.; Raghunathan, R.; Jockusch, S.; Sivaguru, J.* RealizingAzaPaternò-Büchireaction” Angew. Chem.Int. Ed.2017, 56, 7056 –7061.(Frontispiece).